How Do Ponds Actually Work? It's Thanks To These 2 Cycles
All life may have originated from ponds, but how do ponds actually support life? We have the nitrogen and oxygen cycles to thank.
Let’s Nerd Out About Gardening is a twice-monthly publication for curious people who love to nerd out about gardening, farming, and nature. Come nerd out with us researched-backed deep-dives and thoughtfully curated articles delivered straight to your inbox:
Dear Gardenerds,
Last time, we discussed the different ways that we can add a wildlife pond to our gardens, no matter how small, and whether they actually make a difference (spoiler: they do). And how I may have saved my tomatoes from squirrels by just adding a dish of water. (And the squirrel still hasn’t touched them!)
But how do ponds actually work?
How do they maintain themselves?
What is the difference between a healthy pond that supports wildlife and one that’s essentially dead?
Let’s nerd out.

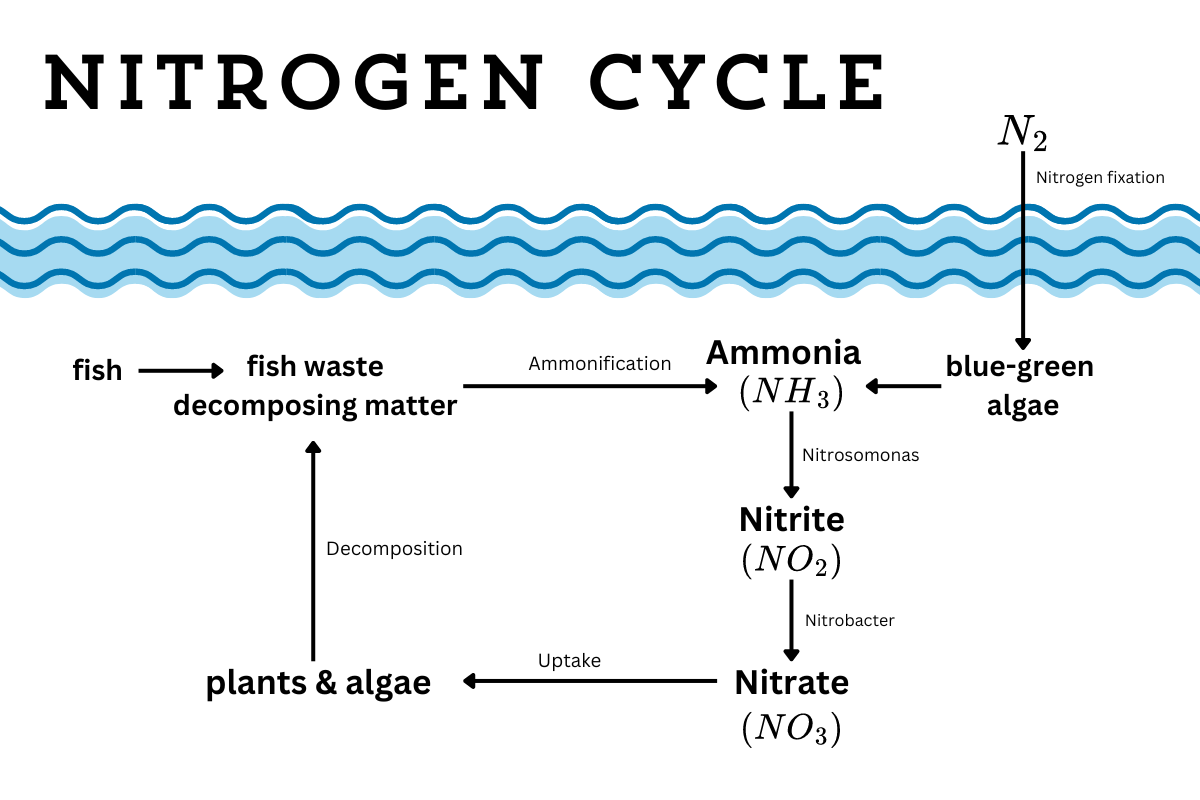
How the nitrogen cycle works
If you have an aquarium, then you probably already know about the nitrogen cycle. As I was trying to get pond fish out of my system, I delved deep into the art of fish keeping. Every beginner source said the same thing — your best chance at keeping fish alive is by knowing how the nitrogen cycle works.
You may also know the nitrogen cycle from gardening or biology class. It’s very similar, but with a few key differences in an aquatic ecosystem.
When you have fish and decaying organic matter, you’re going to have ammonia (NH3) and ammonium (NH4). Plants (and fish food) become ammonia and ammonium as they decay through a process called ammonification. Whether you get more ammonia or more ammonium depends on the pH level of the water. The more acidic, the more likely it is to take on that extra hydrogen molecule.
While we tend to think that the ammonia comes through fish waste, fish actually excrete most of their ammonia from their gills. When ammonia levels rise, their gills get chemical burns. And when their gills are burned, fish struggle to breathe and die. Ammonia is very toxic to fish because of this. (It’s also toxic to other aquatic wildlife) Ammonium is also toxic, but causes less harm, and is safer in slightly higher concentrations.
When ammonia levels appear in a fish tank, you need to do a water change (removing half the water and refilling it with fresh water) to get rid of the extra ammonia. But a natural pond can’t organize a water change. Instead, it has a secret weapon — it has beneficial microbes.
Nitrosomonas bacteria turn ammonia into nitrite (NO2), and then Nitrobacter bacteria turn nitrite into nitrate (NO3). Plants can absorb nitrate, removing it from the water. (This is why aquarium water is so good for plants, or how you can combine fish keeping and hydroponics to grow food without additional fertilizer.)
Now, all three are toxic to fish, but at different levels. No level of ammonia is safe for fish, while fish can live with a bit of nitrite and a bit more nitrate. There’s test kits that you can get that measure each. And if you’re planning to keep fish in your newly developed pond, wait a few weeks to introduce the fish to build up beneficial microbes and get this process going.
The best way to keep all of this in check is by having enough aquatic plants in a pond to soak up the extra nitrates. When planting, you can actually shake off any soil from the plant roots and just weigh the roots down in the water. The extra soil just adds nutrients to the water, and the plants can still take hold.
Alternatively, nitrogen can be stored in sediment as nitrous oxide. While this makes it an interesting solution for nitrogen runoff (see the extra credit reading), it’s not ideal for maintaining a garden pond.
But what happens if nitrogen builds up? After all, an enormous problem in water conservation is all the nitrogen (and phosphorus) runoff from over-fertilizing lawns and from conventional agriculture.
Then, like with weeds covering bare soil, nature takes over.

Algae (phytoplankton) will grow, gorging on the extra nitrogen. If there’s enough nitrogen, not enough competition, and hot temperatures, algae will rapidly reproduce and take over a waterway. When a huge bloom dies, microbes will decompose it — and they’ll need all the lingering oxygen in the water to do so. No oxygen, no life.
(The toxic blue-green algae, while also causing harmful blooms, is not actually algae. It’s a nitrogen fixing bacteria (Cyanobacteria), converting atmospheric nitrogen into ammonia/ammonium. While algae blooms because of excess nitrogen, blue-green algae is caused by phosphorus pollution.)
It’s not just algae, either. Any quickly reproducing plant that floats on the surface and shades out other aquatic plants will do. Michigan has found that storm ponds covered with duckweed have less oxygen and produce more methane.
Which brings us to the other important and arguably more critical cycle in a pond:
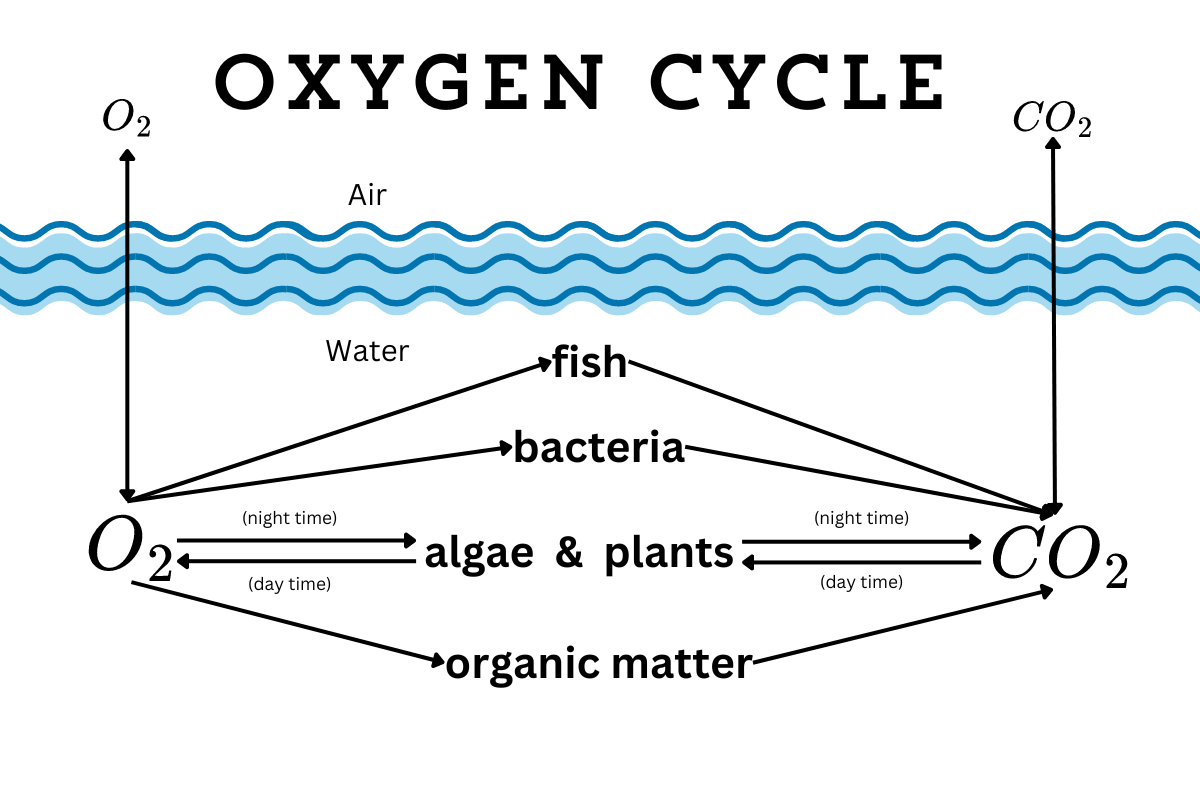
How the oxygen cycle works
They might be underwater, but aquatic creatures still need oxygen to live. Even plants need oxygen around their roots. When there’s no dissolved oxygen in the water, that’s it. Everything dies. So how do ponds get more oxygen?
Water surfaces exchange gases like oxygen and carbon dioxide with the air. When oxygen is low in the water, then oxygen moves from the air to water. If oxygen is high in the water, then it moves to the air.
Colder water holds more oxygen than warmer water. Deeper spots (3 to 4 feet deep) help regulate the temperature, so that water can stay cooler even as temperatures rise.
But it’s not just depth that helps. The more surface area to the water, the more access to oxygen the pond has.
And the more movement on the surface of the water (like by wind, waterfalls, or fountains), the more quickly these gases exchange. This is how a waterfall feature adds oxygen to a pond.
But when the water is covered, whether by plant life or ice, then the process stalls. (Overwintering creatures will slow their heartrate so they need less oxygen. Some pond owners may even add a bubbler during the winter to help them survive.)
Plants can add oxygen to the water too. During the day, when plants are photosynthesizing, they take in carbon dioxide and release oxygen. If the leaves are above the water, then they release oxygen into the atmosphere. If they’re fully submerged, then they release the oxygen back into the water.
But when it gets dark, plants stop photosynthesizing, which means they stop producing oxygen. (They even absorb a little oxygen during the night.) Plant roots are always in need of oxygen, so they continue to pull oxygen from the water.
Meanwhile, everything else in the water (like fish and beneficial microbes) is also absorbing oxygen and replacing it with carbon dioxide all night long. Oxygen levels drop. If they drop too low, then everything suffocates. However, once plants start photosynthesizing again, they start producing oxygen again, and oxygen levels rise.
Except if the next day (or days) is cloudy (plants don’t photosynthesize) and it’s hot (raising the water temperature). That’s when a pond runs into trouble.
Pond fish are pretty good indicators of oxygen levels, as they’re more sensitive and visible than other creatures. An oxygenated pond will have a max of 10 ppm of dissolved oxygen. Fish suffer when they drop to 3ppm and die when they drop to 2ppm. As oxygen levels drop, fish will stay close to the surface where there’s more oxygen (because of the gas exchange).
But fish also make a huge impact on oxygen levels. If there’s too many fish to a pond, then there won’t be enough oxygen for everything (including the fish). Always underestimate how many fish a pond can support, and start off small.
That being said, if a pond has a good amount of open surface area, the water keeps cool, and there are enough plants, there shouldn’t be many problems.
The two processes can seem really intimidating to manage. But nature manages them just fine. It’s just that we humans need to adjust the controls sometimes.
✍ Join the Conversation
What do you think? Did I miss anything?
Do you have a pond? Have you noticed the effects that the nitrogen and oxygen cycles can have on one?
Is there anything that surprised you about these two cycles or maintaining a pond ecosystem?
Share with us in the comments! We want your insight!
Happy growing!
Tanith
P.S. Here’s your extra credit reading!
Building Natural Ponds: Create A Clean, Algae-Free Pond Without Pumps, Filters, Or Chemicals by Robert Pavlis (via New Society Publishers)
If you want to learn more about natural pond design, and the above seems really freaking intimidating, then grab a copy of his book. He’s a Master Gardener that runs GardenMyths.com and a Facebook group on natural ponds, so he really knows his stuff. And he shares how he’s learned to design a natural pond so it just works with little maintenance.
Beaver Ponds With More Sediment Store More Nitrogen, Simple Mapping Reveals (via Advancing Earth and Space Sciences)
As I mentioned above, there’s another way that ponds can deal with nitrogen — bury it in sediment. Beaver ponds can be very good at it, since they can create areas with slow-moving water and lower oxygen that are ideal for this process. The researchers found that just by mapping the zones of beaver ponds by water flow, pond depth, sediment thickness, and sediment size, land managers can get a pretty accurate idea of how much nitrogen is being stored and how much is being passed on down the river without expensive testing.
How beavers are changing Arctic pond and stream microbiology (via National Park Services)
Likewise, researchers have found that the rebound of the beaver population in Alaska have had a profound effect on waterways. Beaver ponds favor aerobic conditions. Tundra lakes and rivers without beavers favored anaerobic conditions, which are more likely to release methane and contain pathogens.
Farm ponds can act as greenhouse gas sinks in the Canadian Prairies by Kerri Finlay and Jackie Webb (via The Conversation)
Conventional farming isn’t that great for the environment, and the Canadian Prairies is pretty much fields of wheat and canola. You’d think that farm ponds here would be pretty polluted. But these two researchers found that 2/3 of the farm ponds they studied actually had low levels of nitrous oxide, particularly when ponds were deeper (at least 3 feet deep). The lower oxygen levels in the deeper regions provides the ideal conditions for aerobic bacteria to consume nitrous oxide. Algae soaks up the rest of the nitrogen.
They also found a weird quirk of the prairies also lowers the carbon dioxide found in lakes and ponds. Our water is alkaline. The pH causes carbon dioxide to be converted into bicarbonate and carbonate.
Deeper ponds also help farmers store more water for our famously dry summers.
Natural ponds remove nitrogen more effectively than stormwater ponds by Brad Buck (via University of Florida IFAS)
Research by PhD student Audrey Goeckner has confirmed that it’s better to have an ecosystem than just water storage when dealing with nitrogen runoff. She confirmed that natural ponds are more likely to remove excess nitrogen from the water while stormwater ponds had the same chance of either removing or storing nitrogen.
Earliest life may have arisen in ponds, not oceans by Jennifer Chu (via MIT News)
We may also have ponds to thank for all life on earth. An MIT study from 2019 believes that ponds are more likely than oceans to be the source of the first life forms, since it was more likely to contain enough nitrogenous oxides to react with other compounds. In deeper bodies of water, like the ocean, nitrogenous oxides would have been too diluted.
Surface Waters: Ammonium is Not Ammonia – Part 1 by John Sawyer (via Iowa State University Extension and Outreach)
While this is an older article from 2008, it also highlights something important — it’s very important to get chemical names right. This article was written about a newspaper article that caused a lot of uproar about high “ammonia” levels in Iowa water systems. As I mentioned above, ammonia is lethal to aquatic life, and ammonium is less so. Ammonium (NH4+) is ionized. Ammonia (NH3) is not. The pH level affects the balance of ammonia and ammonium, and that can fluctuate based on temperature.
It’s actually really hard to test for just ammonia, so laboratories test for ammonium-N plus ammonia-N, and monitor the pH level.
In this case, there was no jump in ammonia. Details matter!